
Chemistry, 16.03.2020 17:50 RicoCheT89
Aluminum sulfate, known as cake alum, has a wide range of uses, from dyeing leather and cloth to purifying sewage. In aqueous solution, it reacts with base to form a white precipitate. (a) Write balanced total and net ionic equations for its reaction with aqueous NaOH. (b) What mass of precipitate forms when 185.5 mL of 0.533 M NaOH is added to 627 mL of a solution that contains 15.8 g of aluminum sulfate per liter?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Aluminum sulfate, known as cake alum, has a wide range of uses, from dyeing leather and cloth to pur...
Questions



Spanish, 18.02.2021 22:30

Mathematics, 18.02.2021 22:30



Mathematics, 18.02.2021 22:30



Mathematics, 18.02.2021 22:30




Mathematics, 18.02.2021 22:30



Law, 18.02.2021 22:30


Mathematics, 18.02.2021 22:30

Mathematics, 18.02.2021 22:30


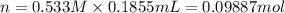
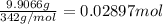
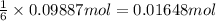
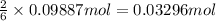 of aluminum hydroxide
of aluminum hydroxide

