
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. You determine that the concentration of the unknown acid was 0.1224 M. You also determined that the acid was monoprotic (HA). What is the K_a and pK_a of your unknown acid

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. Yo...
Questions

Mathematics, 14.07.2020 01:01



Mathematics, 14.07.2020 01:01


Medicine, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Advanced Placement (AP), 14.07.2020 01:01

Advanced Placement (AP), 14.07.2020 01:01



Mathematics, 14.07.2020 01:01







Computers and Technology, 14.07.2020 01:01

 is dissociation constant and the value of
is dissociation constant and the value of 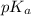 is 4.98.
is 4.98.![pH=-\log[H^+]](/tpl/images/0548/6523/cf945.png)
![2.95=-\log[H^+]](/tpl/images/0548/6523/b4bb5.png)
![[H^+]=10^{-2.95}=0.001122 M](/tpl/images/0548/6523/2ad17.png) ..[1]
..[1]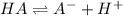
![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0548/6523/a5cb9.png)
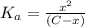
![[H^+]=x =0.001122 M](/tpl/images/0548/6523/90707.png) ( from [1])
( from [1])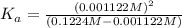
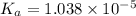
![pK_a=-\log[K_a]](/tpl/images/0548/6523/78bbf.png)
![=-\log[1.038\times 10^{-5}]=4.98](/tpl/images/0548/6523/44380.png)


