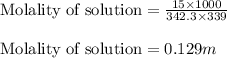Chemistry, 16.03.2020 18:07 angelthompson2018
A student dissolves 15.g of sucrose C12H22O11 in 300.mL of a solvent with a density of 1.13/gmL. The student notices that the volume of the solvent does not change when the sucrose dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
A student dissolves 15.g of sucrose C12H22O11 in 300.mL of a solvent with a density of 1.13/gmL. The...
Questions



Biology, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

Biology, 26.01.2021 19:50


English, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

Chemistry, 26.01.2021 19:50


Mathematics, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

History, 26.01.2021 19:50


Arts, 26.01.2021 19:50

Mathematics, 26.01.2021 19:50

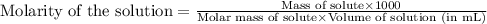
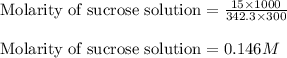
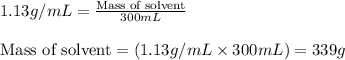
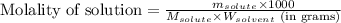
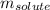 = Given mass of solute (sucrose) = 15 g
= Given mass of solute (sucrose) = 15 g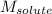 = Molar mass of solute (sucrose) = 342.3 g/mol
= Molar mass of solute (sucrose) = 342.3 g/mol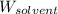 = Mass of solvent = 339 g
= Mass of solvent = 339 g