
Chemistry, 16.03.2020 17:57 graciearany
Given the following chemical reaction, calculate the number of moles of sodium sulfate produced when 3.25 moles of sodium chloride reacts with excess sulfuric acid according to the following balanced reaction; 2 NaCl(aq) + 1 H2SO4(aq) ---> 1 Na2SO4(aq) + 2 HCl(aq)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
You know the right answer?
Given the following chemical reaction, calculate the number of moles of sodium sulfate produced when...
Questions

Mathematics, 29.03.2021 01:00



Computers and Technology, 29.03.2021 01:00

Mathematics, 29.03.2021 01:00




Geography, 29.03.2021 01:00


Chemistry, 29.03.2021 01:00

Health, 29.03.2021 01:00


History, 29.03.2021 01:00

Chemistry, 29.03.2021 01:00

Mathematics, 29.03.2021 01:00




Mathematics, 29.03.2021 01:00

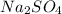 produced is, 1.62 moles
produced is, 1.62 moles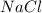 = 3.25 mol
= 3.25 mol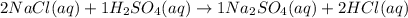
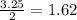 mole of
mole of 

