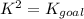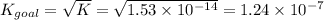Chemistry, 16.03.2020 18:00 mostman077
Determine the value of the equilibrium constant, KgoalKgoalK_goal, for the reaction CO2(g)⇌C(s)+O2(g)CO2(g)⇌C(s)+O2(g), Kgoal=? Kgoal=? by making use of the following information: 1. 2CO2(g)+2H2O(l)⇌CH3COOH(l)+2O2(g)2C O2(g)+2H2O(l)⇌CH3COOH(l)+2O2(g), K1 = K1 = 5.40×10−165.40×10−16 2. 2H2(g)+O2(g)⇌2H2O(l)2H2(g)+O2(g)⇌2H 2O(l), K2 = K2 = 1.06×10101.06×1010 3. CH3COOH(l)⇌2C(s)+2H2(g)+O2(g)CH3COO H(l)⇌2C(s)+2H2(g)+O2(g), K3 = K3 = 2.68×10−92.68×10−9

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Determine the value of the equilibrium constant, KgoalKgoalK_goal, for the reaction CO2(g)⇌C(s)+O2(g...
Questions

English, 23.07.2019 22:00

Chemistry, 23.07.2019 22:00



Biology, 23.07.2019 22:00



Mathematics, 23.07.2019 22:00

English, 23.07.2019 22:00

English, 23.07.2019 22:00


Business, 23.07.2019 22:00


Biology, 23.07.2019 22:00

English, 23.07.2019 22:00

Advanced Placement (AP), 23.07.2019 22:00

English, 23.07.2019 22:00

Computers and Technology, 23.07.2019 22:00


Computers and Technology, 23.07.2019 22:00

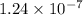 .
.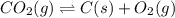
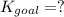
![K_{goal}=\frac{[C][O_2]}{[CO_2]}](/tpl/images/0548/6787/d6c17.png)
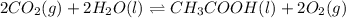 ..[1]
..[1]![K_1=\frac{[CH_3COOH][O_2]^2}{[CO_2]^2[H_2O]^2}](/tpl/images/0548/6787/9e643.png)
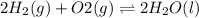 ..[2]
..[2]![K_2=\frac{[H_2O]^2}{[H_2]^2[O_2]}](/tpl/images/0548/6787/e1070.png)
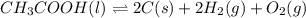 ..[3]
..[3]![K_3=\frac{[C]^2[H_2]^2[O_2]}{[CH_3COOH]}](/tpl/images/0548/6787/ef5ad.png)
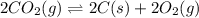

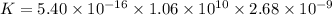
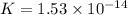
![K=\frac{[C]^2[O_2]^2}{[CO_2]^2}](/tpl/images/0548/6787/82137.png)
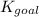 :
: