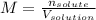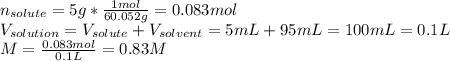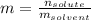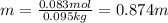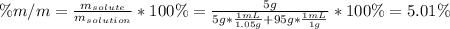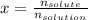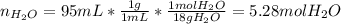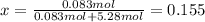Chemistry, 16.03.2020 18:22 cupcake122016
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density of acetic acid is 1.05 g/ml and water is 1.00 g/ml. from this data calculate the concentration of acetic acid in vinegar in: molality, molarity, parts by mass, and the mole fraction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density...
Questions

Computers and Technology, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00

History, 05.11.2020 22:00

History, 05.11.2020 22:00

Mathematics, 05.11.2020 22:00

Biology, 05.11.2020 22:00


Spanish, 05.11.2020 22:00

Biology, 05.11.2020 22:00

Biology, 05.11.2020 22:00

Spanish, 05.11.2020 22:00


Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10

Mathematics, 05.11.2020 22:10


Mathematics, 05.11.2020 22:10