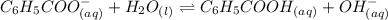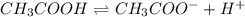Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definitions for acids and bases.
1) An Arrhenius acid is a substance that dissolves in water to produce H+ or H3O+.
2) A Br∅nsted-Lowry base is a proton acceptor.
3) CH3COOH is an Arrhenius base.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definition...
Questions


History, 19.03.2020 00:57

Mathematics, 19.03.2020 00:57










History, 19.03.2020 00:57







 .
.