
Chemistry, 16.03.2020 18:56 pierrezonra
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration was found to be 4.081. If the initial pH of the weak acid solution (before titration) has a pH of 2.348, what was the concentration of the weak acid solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1

You know the right answer?
An unknown weak acid, HA, it titrated with 1.2 M NaOH. The pH at the halfway point of this titration...
Questions


History, 25.09.2021 01:30


English, 25.09.2021 01:30

Mathematics, 25.09.2021 01:40

Mathematics, 25.09.2021 01:40


Mathematics, 25.09.2021 01:40



Chemistry, 25.09.2021 01:40

Mathematics, 25.09.2021 01:40

Mathematics, 25.09.2021 01:40


Mathematics, 25.09.2021 01:40




Mathematics, 25.09.2021 01:40

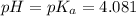 (At halfway point)
(At halfway point)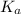 , we use the equation:
, we use the equation: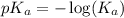
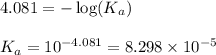
![pH=-\log [H^+]](/tpl/images/0548/8236/37e81.png)
![2.348=-\log [H^+]](/tpl/images/0548/8236/8ea67.png)
![[H^+]=10^{-2.348}=4.487\times 10^{-3}](/tpl/images/0548/8236/4ae47.png)
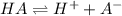
![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0548/8236/66f51.png)
![[H^+]=[A^-]=4.487\times 10^{-3}](/tpl/images/0548/8236/32c77.png)
![8.298\times 10^{-5}=\frac{(4.487\times 10^{-3})\times (4.487\times 10^{-3})}{[HA]}](/tpl/images/0548/8236/82702.png)
![[HA]=0.243M](/tpl/images/0548/8236/b588d.png)


