Chemistry, 16.03.2020 19:25 corbeansbrain
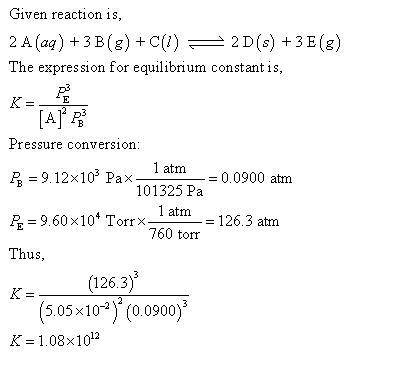
For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) 2A(aq)+3B(g)+C(l)↽−−⇀2D(s)+3E(g) the concentrations and pressures at equilibrium are [ A ] = 5.66 × 10 − 2 M [A]=5.66×10−2 M , P B = 5.69 × 10 3 Pa PB=5.69×103 Pa , [ C ] = 9.14 M [C]=9.14 M , [ D ] = 15.78 M [D]=15.78 M , and P E = 9.33 × 10 4 torr PE=9.33×104 torr . Calculate the thermodynamic equilibrium constant, K K .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
For this heterogeneous system 2 A ( aq ) + 3 B ( g ) + C ( l ) − ⇀ ↽ − 2 D ( s ) + 3 E ( g ) 2A(aq)+...
Questions



Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30


Biology, 21.10.2019 13:30

Chemistry, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30


English, 21.10.2019 13:30



Spanish, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30


History, 21.10.2019 13:30

History, 21.10.2019 13:30