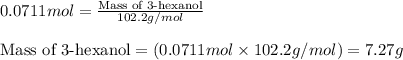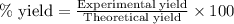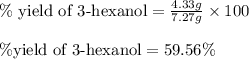4.33 g of 3-hexanol were obtained from 5.84 g of hex-3-ene. Determine the percentage yield of 3-hexanol. a Determine the moles of hex-3-ene, , used in the experiment. (To avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.) Moles of hex-3-ene used

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
4.33 g of 3-hexanol were obtained from 5.84 g of hex-3-ene. Determine the percentage yield of 3-hexa...
Questions

Geography, 16.04.2020 05:37


History, 16.04.2020 05:37

Mathematics, 16.04.2020 05:37


Mathematics, 16.04.2020 05:37


Mathematics, 16.04.2020 05:37


Mathematics, 16.04.2020 05:38

Mathematics, 16.04.2020 05:38





English, 16.04.2020 05:38

Mathematics, 16.04.2020 05:38

Mathematics, 16.04.2020 05:38

Mathematics, 16.04.2020 05:38

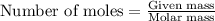 .....(1)
.....(1)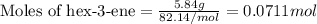
![\text{hex-3-ene}+H_2O\xrightarrow []{10\% H_2SO_4} \text{3-hexanol}](/tpl/images/0548/9898/4b79c.png)
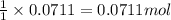 of 3-hexanol
of 3-hexanol