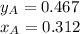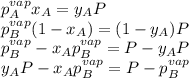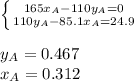Chemistry, 16.03.2020 20:07 minersaysay22
Two volatile liquids A (P A pure= 165 Torr) and B (PB pure= 85.1 Torr) are confined in a piston/cylinder assembly. Initially only the liquid phase is present. As the external pressure is reduced, vapor is first observed at a total pressure of 110 Torr. Calculate the mole fraction of component A in the solution (XA) and the mole fraction of component A in the vapor (YA).

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Two volatile liquids A (P A pure= 165 Torr) and B (PB pure= 85.1 Torr) are confined in a piston/cyli...
Questions


Mathematics, 09.06.2021 16:50


Mathematics, 09.06.2021 16:50



Mathematics, 09.06.2021 16:50

English, 09.06.2021 16:50

Mathematics, 09.06.2021 16:50


Mathematics, 09.06.2021 16:50


Mathematics, 09.06.2021 16:50



Mathematics, 09.06.2021 16:50


Mathematics, 09.06.2021 16:50