
Chemistry, 16.03.2020 20:09 ginareyes0423
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the following reactions and given ΔHΔH values: C3H8(l)+5O2(g)→3CO2(g)+4H2O(g),ΔHC( s)+O2(g)→CO2(g),ΔH2H2(g)+O2(g)→2H2O (g),ΔH===−2026.6kJ−393.5kJ−483.5kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

You know the right answer?
Calculate ΔHrxnΔHrxn for the following reaction: 3C(s)+4H2(g)→C3H8(l)3C(s)+4H2(g)→C3 H8(l) Use the f...
Questions

Social Studies, 30.07.2019 14:00


Social Studies, 30.07.2019 14:00


History, 30.07.2019 14:00





Biology, 30.07.2019 14:00

Business, 30.07.2019 14:00

Social Studies, 30.07.2019 14:00


Geography, 30.07.2019 14:00

Mathematics, 30.07.2019 14:00


History, 30.07.2019 14:00


Business, 30.07.2019 14:00

Social Studies, 30.07.2019 14:00

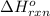 for the reaction is -120.9 kJ.
for the reaction is -120.9 kJ.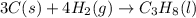
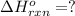
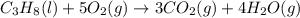
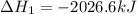
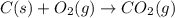
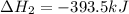 ( × 3)
( × 3)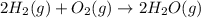
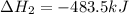 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0548/9833/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times -(-2026.6))+(3\times (-393.5))+(2\times (-483.5))]=-120.9kJ](/tpl/images/0548/9833/4ea2a.png)


