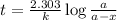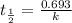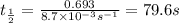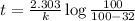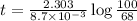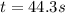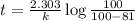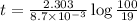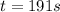Chemistry, 16.03.2020 20:20 AaronEarlMerringer
Acetone is one of the most important solvents in organic chemistry. It is used to dissolve everything from fats and waxes to airplane glue and nail polish. At high temperatures, it decomposes in a first-order process to methane and ketene (CH2═C═O). At 600°C, the rate constant is 8.7 × 10^−3 s^−1.
a. What is the half-life of the reaction?
b. How much time is required for 32% of a sample of acetone to decompose?
c. How much time is required for 81% of a sample of acetone to decompose?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
Acetone is one of the most important solvents in organic chemistry. It is used to dissolve everythin...
Questions




History, 19.12.2019 01:31




English, 19.12.2019 01:31

Biology, 19.12.2019 01:31

Mathematics, 19.12.2019 01:31

Mathematics, 19.12.2019 01:31





Mathematics, 19.12.2019 01:31


History, 19.12.2019 01:31