Show Your Work
2SO3(g) -> 2SO2(g)+O2(g)
Calculate Keq for this reaction if the eq...

Chemistry, 16.03.2020 20:43 lelseymota123
Show Your Work
2SO3(g) -> 2SO2(g)+O2(g)
Calculate Keq for this reaction if the equalibrium concentrations are: |SO2|=0.42M; |O2|=0.21M; |SO3|=0.072M

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Questions

Physics, 12.08.2020 08:01

Computers and Technology, 12.08.2020 08:01

History, 12.08.2020 08:01



Biology, 12.08.2020 08:01





Mathematics, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01


History, 12.08.2020 08:01


Physics, 12.08.2020 08:01



![\left [ SO_{2} \right ] = 0.42 \textrm{ M}, \left [ O_{2} \right ] = 0.21 \textrm{ M}, \left [ SO_{3} \right ] = 0.072 \textrm{ M}](/tpl/images/0549/0905/fc0fc.png) \\
\\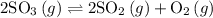
![K_{eq} = \displaystyle \frac{\left [ SO_{2} \right ]^{2}\times \left [ O_{2} \right ]}{\left [ SO_{3} \right ]^{2}} \\K_{eq} = \displaystyle \frac{\left ( 0.42 \right )^{2}\times 0.21}{\left ( 0.072 \right )^{2}} \\K_{eq} = 7.145](/tpl/images/0549/0905/2d355.png)
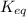 for the reaction is 7.145
for the reaction is 7.145 

