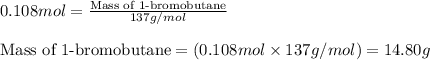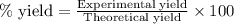Chemistry, 16.03.2020 20:53 kfloyd6046
A student isolated 7.2 g of 1-bromobutane reacting equimolar amounts of 1-butanol (10 ml) and NaBr (11.1 g) in the presence of sulfuric acid. The yield of the reaction is Select one: a. 95 % b. 48 % c. 84% d. 72 %

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
A student isolated 7.2 g of 1-bromobutane reacting equimolar amounts of 1-butanol (10 ml) and NaBr (...
Questions

Mathematics, 07.09.2021 20:50

Mathematics, 07.09.2021 20:50

History, 07.09.2021 20:50

Mathematics, 07.09.2021 20:50

Mathematics, 07.09.2021 20:50



Mathematics, 07.09.2021 20:50

Social Studies, 07.09.2021 20:50


World Languages, 07.09.2021 20:50


Arts, 07.09.2021 20:50

Mathematics, 07.09.2021 21:00


History, 07.09.2021 21:00

History, 07.09.2021 21:00


Mathematics, 07.09.2021 21:00

Mathematics, 07.09.2021 21:00

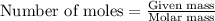 .....(1)
.....(1)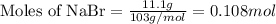
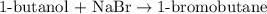
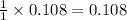 moles of 1-bromobutane
moles of 1-bromobutane