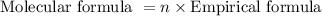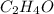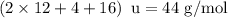Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3
You know the right answer?
The empirical formula of an organic compound is C2H4O. The molecular mass of the compound is 176g/mo...
Questions

Biology, 25.08.2021 18:00

Mathematics, 25.08.2021 18:00

Social Studies, 25.08.2021 18:00

Geography, 25.08.2021 18:00

Mathematics, 25.08.2021 18:00

History, 25.08.2021 18:00

Mathematics, 25.08.2021 18:00

Business, 25.08.2021 18:00

History, 25.08.2021 18:00






Physics, 25.08.2021 18:00

Mathematics, 25.08.2021 18:00



Mathematics, 25.08.2021 18:00

World Languages, 25.08.2021 18:00

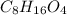 . The molecular formula is obtained by the following expression shown below
. The molecular formula is obtained by the following expression shown below