
Place the following substances in order of increasing boiling point. CH3CH2CH2OH Ne CH3OCH3 Place the following substances in order of increasing boiling point. CH3CH2CH2OH Ne CH3OCH3 CH3OCH3 < Ne < CH3CH2CH2OH CH3CH2CH2OH < CH3OCH3 < Ne Ne < CH3OCH3 < CH3CH2CH2OH CH3CH2CH2OH < Ne < CH3OCH3 Ne < CH3CH2CH2OH < CH3OCH3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1
You know the right answer?
Place the following substances in order of increasing boiling point. CH3CH2CH2OH Ne CH3OCH3 Place th...
Questions

Mathematics, 26.05.2021 01:30



Mathematics, 26.05.2021 01:30





Mathematics, 26.05.2021 01:30



Biology, 26.05.2021 01:30


History, 26.05.2021 01:30






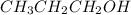 there will be strong dipole-dipole interactions because this compound has hydrogen bonding. Whereas in
there will be strong dipole-dipole interactions because this compound has hydrogen bonding. Whereas in 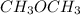 there is no hydrogen bonding taking place.
there is no hydrogen bonding taking place.

