
Chemistry, 16.03.2020 22:21 lerasteidl
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105 ) b. 0.100 M sodium propanoate (NaC3H5O2) c. pure H2O d. a mixture containing 0.100 M HC3H5O2 and 0.100 M NaC3H5O2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1


Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105...
Questions

Computers and Technology, 23.04.2020 14:11


Mathematics, 23.04.2020 14:12


Biology, 23.04.2020 14:12

Geography, 23.04.2020 14:12

Mathematics, 23.04.2020 14:12


English, 23.04.2020 14:12

Biology, 23.04.2020 14:12



Mathematics, 23.04.2020 14:13

Mathematics, 23.04.2020 14:13

History, 23.04.2020 14:13

Mathematics, 23.04.2020 14:13

Physics, 23.04.2020 14:13

Mathematics, 23.04.2020 14:14


![K_{a} = \frac{[C_{3}H_{5}O_{2}^{-}][H_{3}O^{+}]}{[C_{3}H_{6}O_{2}]}](/tpl/images/0549/3755/fa55a.png)
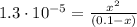 (2)
(2)![pH = -log [H_{3}O^{+}] = -log (0.00113) = 2.95](/tpl/images/0549/3755/8dc10.png)
![K_{b} = \frac{[C_{3}H_{6}O_{2}][OH^{-}]}{[C_{3}H_{5}O_{2}^{-}]}](/tpl/images/0549/3755/eac92.png)
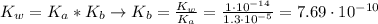
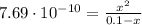 (3)
(3)![pOH = -log[OH^{-}] = -log(8.77\cdot 10^{-6}) = 5.06 \rightarrow pH = 14 - pOH = 8.94](/tpl/images/0549/3755/13708.png)
![K_{w} = [H^{+}][OH^{-}] \rightarrow 1\cdot 10^{-14} = [H^{+}][OH^{-}]](/tpl/images/0549/3755/f80a5.png)
![1\cdot 10^{-14} = [H^{+}]^{2} \rightarrow [H^{+}] = \sqrt{1\cdot 10^{-14}} = 1 \cdot 10^{-7}](/tpl/images/0549/3755/97d56.png)
![pH = -log [H^{+}] = -log (1 \cdot 10^{-7}) = 7.00](/tpl/images/0549/3755/acfd0.png)
![pH = pKa + log(\frac{[C_{3}H_{5}O_{2}^{-}]}{[C_{3}H_{6}O_{2}]}) = -log(1.3 \cdot 10^{-5}) + log(\frac{0.1}{0.1}) = 4.89](/tpl/images/0549/3755/7ff66.png)


