
Chemistry, 16.03.2020 22:34 McGregorshamara
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify the state of each reactant and product. + Is iron(III) nitrate considered soluble or not soluble ? ... ... A. Soluble ... B. Not soluble Based upon this, the equilibrium constant for this reaction will be: ... ... A. Greater than 1 ... B. Less than 1

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3


Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2
You know the right answer?
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify th...
Questions







Biology, 29.02.2020 04:41



Mathematics, 29.02.2020 04:41








Mathematics, 29.02.2020 04:42


Mathematics, 29.02.2020 04:42

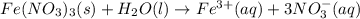
![K_{eq} = [Fe^{3+}][(NO_{3})_{3}]^{3}](/tpl/images/0549/4240/1e6b7.png)


