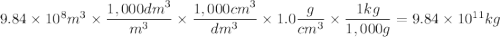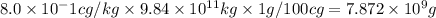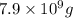Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
The sea water has 8.0x10^-1 cg of element strontium. Assuming that all strontium could be recovered,...
Questions


History, 12.02.2020 04:55

Mathematics, 12.02.2020 04:55

Biology, 12.02.2020 04:55




Computers and Technology, 12.02.2020 04:55




Mathematics, 12.02.2020 04:55

Mathematics, 12.02.2020 04:55


Computers and Technology, 12.02.2020 04:55





History, 12.02.2020 04:55