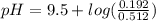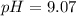Chemistry, 16.03.2020 23:01 thanks5640
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochloric acid are added to 125 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochl...
Questions

Mathematics, 25.02.2020 17:59








Social Studies, 25.02.2020 17:59

History, 25.02.2020 17:59

Mathematics, 25.02.2020 17:59



Mathematics, 25.02.2020 17:59

Mathematics, 25.02.2020 17:59



Mathematics, 25.02.2020 17:59


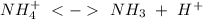
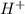 of the hydrochloric acid (
of the hydrochloric acid (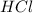 ) will interact with the base of the buffer system (
) will interact with the base of the buffer system (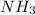 ) to produce more acid (
) to produce more acid (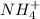 ), so:
), so: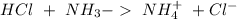
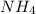 will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So:
will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So: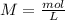
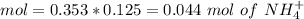
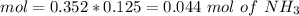
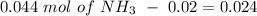
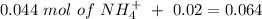
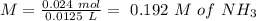
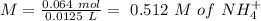
![pH=p{ K }_{ a }+log(\frac { { [A }^{ - }] }{ [HA] } )](/tpl/images/0549/5131/c1f49.png)