Chemistry, 16.03.2020 22:55 brendacauani12345
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1.01g/mL, what is the heat of the calorimeter in kJ given the temperature change of the above equation. You may assume the solution has a heat capacity of 4.180J/gK. Express your final answer in kJ and with 2 decimal places

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1....
Questions

SAT, 05.02.2021 03:00


History, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00


Mathematics, 05.02.2021 03:00



Social Studies, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

Spanish, 05.02.2021 03:00



Chemistry, 05.02.2021 03:00



Mathematics, 05.02.2021 03:00


Spanish, 05.02.2021 03:00

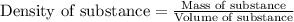
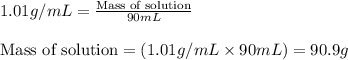
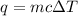
 = change in temperature = -5.5 K
= change in temperature = -5.5 K