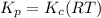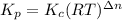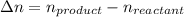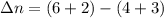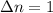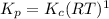Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that shows how to calculate from for this reaction at an absolute temperature . You can assume is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Consider the following chemical equilibrium: 4NH3+3O2=2N2+6H2O Now write an equation below that show...
Questions

English, 13.10.2019 15:50





Geography, 13.10.2019 15:50

History, 13.10.2019 15:50




Mathematics, 13.10.2019 15:50

English, 13.10.2019 15:50

Social Studies, 13.10.2019 15:50




Mathematics, 13.10.2019 15:50


Mathematics, 13.10.2019 15:50

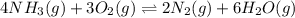
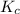 will be,
will be,![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0549/5664/b62d0.png)
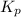 will be,
will be,