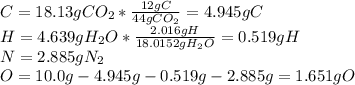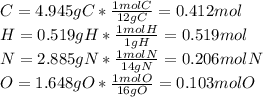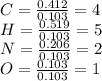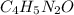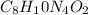Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Caffeine, a molecule found in coffee, tea, and certain soft drinks, contains C, H, O, and N. Combust...
Questions


History, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30


Mathematics, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30

Physics, 12.09.2019 04:30


Computers and Technology, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30

History, 12.09.2019 04:30


History, 12.09.2019 04:30

Physics, 12.09.2019 04:30

English, 12.09.2019 04:30

Biology, 12.09.2019 04:30

History, 12.09.2019 04:30



Social Studies, 12.09.2019 04:30