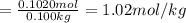Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
A solution of phosphoric acid was made by dissolving 10.0 g of H3PO4 in 100.0 mL of water. The resul...
Questions

Mathematics, 23.10.2019 18:50




Biology, 23.10.2019 18:50


Computers and Technology, 23.10.2019 18:50

Arts, 23.10.2019 18:50

Biology, 23.10.2019 18:50

History, 23.10.2019 18:50

Computers and Technology, 23.10.2019 18:50


Mathematics, 23.10.2019 18:50

Mathematics, 23.10.2019 18:50



History, 23.10.2019 18:50


Mathematics, 23.10.2019 18:50

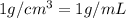
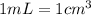
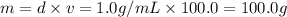
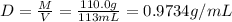
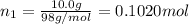
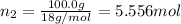
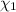
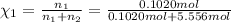
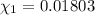
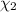
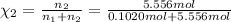
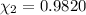
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0549/6266/0dac6.png)
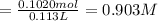
![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](/tpl/images/0549/6266/71fd2.png)