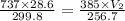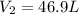A weather balloon is inflated to a volume of 28.6 L at a pressure of 737 mmHg and a temperature of 26.8 ∘C. The balloon rises in the atmosphere to an altitude where the pressure is 385 mmHg and the temperature is -16.3 ∘C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
A weather balloon is inflated to a volume of 28.6 L at a pressure of 737 mmHg and a temperature of 2...
Questions

Advanced Placement (AP), 28.05.2020 20:08


Mathematics, 28.05.2020 20:08

Mathematics, 28.05.2020 20:08


English, 28.05.2020 20:08


Mathematics, 28.05.2020 20:08

Mathematics, 28.05.2020 20:08



Biology, 28.05.2020 20:08

Mathematics, 28.05.2020 20:08

Mathematics, 28.05.2020 20:08


Mathematics, 28.05.2020 20:08



Mathematics, 28.05.2020 20:08

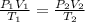
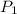 = initial pressure of gas = 737 mm Hg
= initial pressure of gas = 737 mm Hg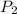 = final pressure of gas = 385 mm Hg
= final pressure of gas = 385 mm Hg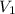 = initial volume of gas = 28.6 L
= initial volume of gas = 28.6 L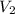 = final volume of gas = ?
= final volume of gas = ?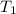 = initial temperature of gas =
= initial temperature of gas = 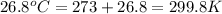
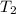 = final temperature of gas =
= final temperature of gas =