
Chemistry, 17.03.2020 01:09 haileeattaway
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO2 + NO2 → NO + NO3 step 2: NO3 + CO → NO2 + CO2 The experimental rate law is rate = k[NO2]2 (a) Write the equation for the overall reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
The reaction between NO2 and CO to produce NO and CO2 is believed to occur via two steps: step 1: NO...
Questions







Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40



Advanced Placement (AP), 17.12.2020 06:40

Geography, 17.12.2020 06:40


Mathematics, 17.12.2020 06:40


Health, 17.12.2020 06:40


World Languages, 17.12.2020 06:40

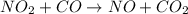
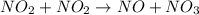
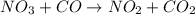
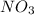 is produced in the first step and subsequently consumed in second step. Hence,
is produced in the first step and subsequently consumed in second step. Hence, 

