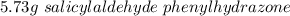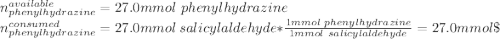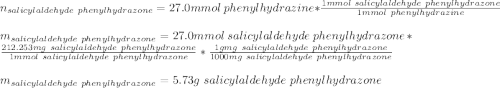Chemistry, 17.03.2020 03:14 michellelirett
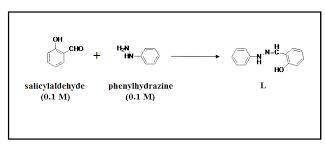
A 2.92 g (27.0 mmol) sample of phenylhydrazine was dissolved in 10 mL of 95% ethanol and stirred while 3.30 g (27.0 mmol) of salicylaldehyde, dissolved in 15 mL of ethanol, was added. what is the theoretical yield in grams for salicylaldehyde phenylhydrazone?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
A 2.92 g (27.0 mmol) sample of phenylhydrazine was dissolved in 10 mL of 95% ethanol and stirred whi...
Questions

Biology, 29.05.2021 16:30




Chemistry, 29.05.2021 16:30

Social Studies, 29.05.2021 16:30





Mathematics, 29.05.2021 16:30



Mathematics, 29.05.2021 16:30

Social Studies, 29.05.2021 16:30


English, 29.05.2021 16:30


Mathematics, 29.05.2021 16:30

Physics, 29.05.2021 16:30