
Chemistry, 17.03.2020 03:18 violetagamez2
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( aq ) . What will be the concentration of Ca 2 + ( aq ) when Ag 2 SO 4 ( s ) begins to precipitate? Solubility-product constants, K sp , can be found in the chempendix.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

You know the right answer?
Sodium sulfate is slowly added to a solution containing 0.0500 M Ca 2 + ( aq ) and 0.0300 M Ag + ( a...
Questions





Mathematics, 10.12.2020 22:20

English, 10.12.2020 22:20



History, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20


Arts, 10.12.2020 22:20


Biology, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20

Spanish, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

![[Ca^{2+}]](/tpl/images/0550/0535/17576.png) ion is, 0.00371 M
ion is, 0.00371 M![[SO_4^{2-}]](/tpl/images/0550/0535/43b69.png) ion.
ion.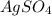 will be:
will be: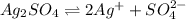
![K_{sp}=[Ag^{+}]^2[SO_4^{2-}]](/tpl/images/0550/0535/80963.png)
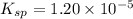
![1.20\times 10^{-5}=(0.0300)^2\times [SO_4^{2-}]](/tpl/images/0550/0535/d8931.png)
![[SO_4^{2-}]=0.0133M](/tpl/images/0550/0535/cd404.png)
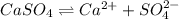
![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0550/0535/958f2.png)
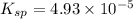
![4.93\times 10^{-5}=[Ca^{2+}]\times (0.0133)](/tpl/images/0550/0535/7fa1a.png)
![[Ca^{2+}]=0.00371M](/tpl/images/0550/0535/7d329.png)


