
Chemistry, 17.03.2020 04:05 lilrel8602
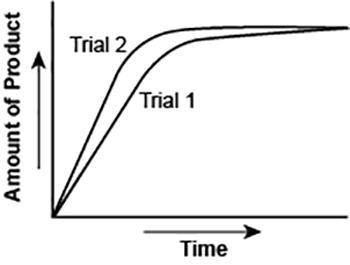
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A. Trial 1, because the average rate of the reaction is lower.
B. Trial 1, because this reaction lasted for a longer duration than Trial 2.
C. Trial 2, because this reaction was initially fast and later slowed down.
D. Trial 2, because the volume of product formed per unit time was higher.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions



Mathematics, 17.01.2020 07:31

Mathematics, 17.01.2020 07:31



English, 17.01.2020 07:31

History, 17.01.2020 07:31

English, 17.01.2020 07:31

Mathematics, 17.01.2020 07:31

Mathematics, 17.01.2020 07:31

Mathematics, 17.01.2020 07:31

Mathematics, 17.01.2020 07:31


Health, 17.01.2020 07:31

English, 17.01.2020 07:31




Mathematics, 17.01.2020 07:31




