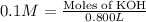Chemistry, 17.03.2020 04:19 theodisb8440
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this in three steps:
Fill a volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
A chemist must prepare 800ml of potassium hydroxide solution with a pH 13 of at 25. He will do this...
Questions

Biology, 23.11.2020 21:20


History, 23.11.2020 21:30

Arts, 23.11.2020 21:30

Social Studies, 23.11.2020 21:30


History, 23.11.2020 21:30


Mathematics, 23.11.2020 21:30




Mathematics, 23.11.2020 21:30

History, 23.11.2020 21:30

Engineering, 23.11.2020 21:30


History, 23.11.2020 21:30

Mathematics, 23.11.2020 21:30


Geography, 23.11.2020 21:30

![pOH=-\log[OH^-]](/tpl/images/0550/1622/fe336.png)
![1=-\log[OH^-]](/tpl/images/0550/1622/3f830.png)
![[OH^-]=0.1 M](/tpl/images/0550/1622/e907d.png)
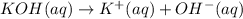
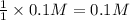 of KOH
of KOH![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0550/1622/0dac6.png)