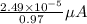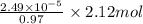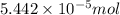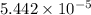Chemistry, 17.03.2020 04:40 hannahking1869
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon juice sample. A detector signal of 2.12 uA was observed. A standard addition of 1.00 mL of 24.9 mM ascorbic acid standard increased the current to 3.09 uA. Find the concentration of vitamin C in the watermelon juice.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon...
Questions

History, 03.08.2019 10:00

English, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00


English, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00

History, 03.08.2019 10:00

History, 03.08.2019 10:00


Social Studies, 03.08.2019 10:00


History, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00

Mathematics, 03.08.2019 10:00






Mathematics, 03.08.2019 10:00

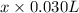
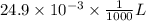
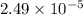 mole
mole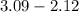

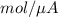 will be equal to as follows.
will be equal to as follows.