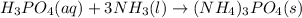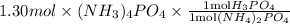Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of phosphoric acid needed to produce of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting...
Questions

Mathematics, 02.10.2019 22:30

Social Studies, 02.10.2019 22:30

Biology, 02.10.2019 22:30


Mathematics, 02.10.2019 22:30


Biology, 02.10.2019 22:30

History, 02.10.2019 22:30

Business, 02.10.2019 22:30

Spanish, 02.10.2019 22:30


Mathematics, 02.10.2019 22:30

Mathematics, 02.10.2019 22:30

English, 02.10.2019 22:30


History, 02.10.2019 22:30

History, 02.10.2019 22:30


Mathematics, 02.10.2019 22:30

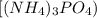 is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid
is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid 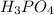 with liquid ammonia. Calculate the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
with liquid ammonia. Calculate the moles of phosphoric acid needed to produce 1.30 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.