
Calculate the pH at the halfway point and at the equivalence point for each of the following titrations. 100.0 mL of 0.60 M titrated by 0.10 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.20 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.15 M pH at the halfway point = pH at the equivalence point ='

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Calculate the pH at the halfway point and at the equivalence point for each of the following titrati...
Questions




Physics, 14.07.2021 06:30


English, 14.07.2021 06:30



Chemistry, 14.07.2021 06:40


Mathematics, 14.07.2021 06:40

Mathematics, 14.07.2021 06:40

Mathematics, 14.07.2021 06:40


Mathematics, 14.07.2021 06:40

Mathematics, 14.07.2021 06:40


Chemistry, 14.07.2021 06:40

History, 14.07.2021 06:40

Mathematics, 14.07.2021 06:40

 (
( ) titrated by 0.10 M
) titrated by 0.10 M 
 .
. →
→ 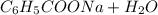
 + log
+ log 
 = 4.20
= 4.20

