
Chemistry, 17.03.2020 05:26 mixedkiddo
A decorative "ice" sculpture is carved from dry ice (solid CO2) and held at its sublimation point of –78.5°C. Consider the process in which a CO2 sculpture, weighing 389 g, sublimes on a granite tabletop. The temperature of the granite is 12.0°C and the process occurs reversibly. Assume a final temperature for the CO2 vapor of –78.5°C. The enthalpy of sublimation of CO2 is 26.1 kJ/mol. a. Calculate the entropy of sublimation for carbon dioxide (the system) ___ J/Kmol b. Calculate the entropy of the universe for this reversible process. Use three significant figures in the answer __J/K

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1


Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
A decorative "ice" sculpture is carved from dry ice (solid CO2) and held at its sublimation point of...
Questions

Mathematics, 16.12.2019 18:31

Physics, 16.12.2019 18:31

Mathematics, 16.12.2019 18:31



Mathematics, 16.12.2019 18:31

Mathematics, 16.12.2019 18:31

Arts, 16.12.2019 18:31

Mathematics, 16.12.2019 18:31






English, 16.12.2019 18:31

English, 16.12.2019 18:31

Mathematics, 16.12.2019 18:31


Social Studies, 16.12.2019 18:31

Mathematics, 16.12.2019 18:31

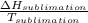
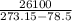
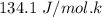
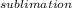 =
= 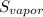 - S
- S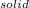 =
= 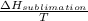
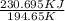
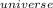 =
= 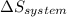 + ΔS
+ ΔS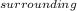
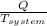
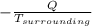
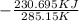 = 1.185 KJ/K - 0.809 KJ/K = 0.376 KJ/K
= 1.185 KJ/K - 0.809 KJ/K = 0.376 KJ/K

