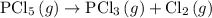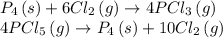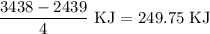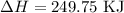Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: PCl5(g)...
Questions

Mathematics, 24.04.2021 02:00

Mathematics, 24.04.2021 02:00

Mathematics, 24.04.2021 02:00


Mathematics, 24.04.2021 02:00



Medicine, 24.04.2021 02:00

English, 24.04.2021 02:00


English, 24.04.2021 02:00






History, 24.04.2021 02:00

Mathematics, 24.04.2021 02:00

Social Studies, 24.04.2021 02:00

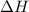 for the desired reaction will be 249.75 KJ.
for the desired reaction will be 249.75 KJ.