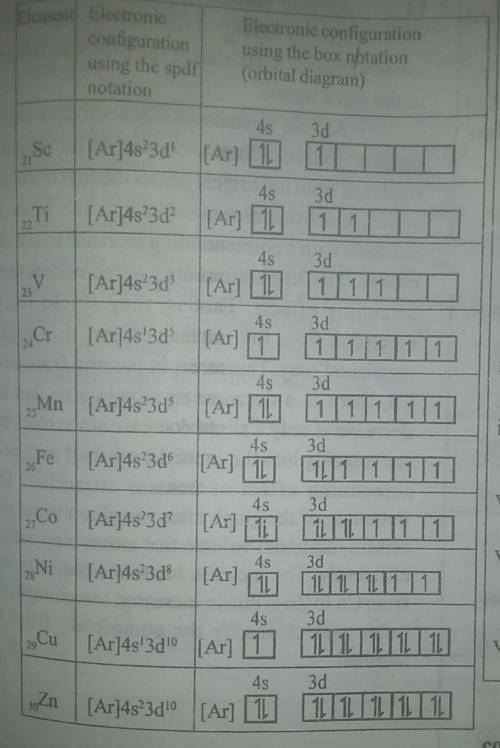
7. (04wP2-5)Elements with atomic number 21 to 30 are d-block elements.
(a) Identify which of t...

Chemistry, 17.03.2020 10:59 chloeethoma24
7. (04wP2-5)Elements with atomic number 21 to 30 are d-block elements.
(a) Identify which of these elements are not considered to be typical transition elements.
12
sh
(b) Complex ions consist of a central metal ion surrounded by ligands. Define the term ligand.
12
(c) Complete the table below to show the oxidation state of the transition element.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
Questions






Mathematics, 06.05.2021 07:00

Chemistry, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00




Biology, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00


Mathematics, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00

Mathematics, 06.05.2021 07:00

Biology, 06.05.2021 07:00

Physics, 06.05.2021 07:00