
Chemistry, 17.03.2020 17:12 joshuahagerman1404
A chemistry student needs of heptane for an experiment. He has available of a w/w solution of heptane in chloroform. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
A chemistry student needs of heptane for an experiment. He has available of a w/w solution of heptan...
Questions

Mathematics, 15.01.2021 20:40




Mathematics, 15.01.2021 20:40




Mathematics, 15.01.2021 20:40

Spanish, 15.01.2021 20:40



Mathematics, 15.01.2021 20:40

Mathematics, 15.01.2021 20:40


English, 15.01.2021 20:40

Biology, 15.01.2021 20:40

Mathematics, 15.01.2021 20:40


Biology, 15.01.2021 20:40

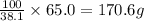 of solution
of solution

