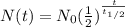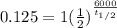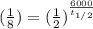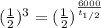Chemistry, 17.03.2020 17:42 whrjegt4jrnfdvj
A radioactive isotope, 14C decays to become 14N. After a time period of about 6,000 years, only about 12.5% of an original sample of 14C remains. The remainder has decayed to 14N. According to this information, approximately how long is one half-life of 14C?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
A radioactive isotope, 14C decays to become 14N. After a time period of about 6,000 years, only abou...
Questions


Physics, 17.08.2020 07:01

Mathematics, 17.08.2020 07:01

Mathematics, 17.08.2020 07:01

Biology, 17.08.2020 07:01

Social Studies, 17.08.2020 07:01

Mathematics, 17.08.2020 07:01


Mathematics, 17.08.2020 07:01

Mathematics, 17.08.2020 07:01



Mathematics, 17.08.2020 07:01


English, 17.08.2020 07:01



Physics, 17.08.2020 07:01