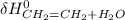Chemistry, 17.03.2020 19:23 alwayspouty6438
Industrial ethanol (CH3CH2OH) is produced by a catalytic reaction of ethylene (CH2═CH2) with water at high pressures and temperatures. Calculate ΔH o rxn for this gas-phase hydration of ethylene to ethanol, using bond energies and then using enthalpies of formation.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Industrial ethanol (CH3CH2OH) is produced by a catalytic reaction of ethylene (CH2═CH2) with water a...
Questions

Social Studies, 31.07.2019 02:10


Medicine, 31.07.2019 02:10



Medicine, 31.07.2019 02:10

Health, 31.07.2019 02:10












German, 31.07.2019 02:10

 ∑ energy of old bond breaking + ∑ energies of the new bond formation.
∑ energy of old bond breaking + ∑ energies of the new bond formation.![[(4 * BE_{C-H}) + BE_{C-C}) + + BE_{O-H})]](/tpl/images/0550/7790/369dd.png) + ∑
+ ∑ ![[(5 * BE_{C-H}) + BE_{C-C}) + BE_{O-H}+ BE_{C-O})]](/tpl/images/0550/7790/f8d51.png)
![[4*413 kJ)+(614kJ)+(2*647kJ]](/tpl/images/0550/7790/b75fe.png) + ∑
+ ∑ ![(5*-413kJ)+(-347kJ)+(-467kJ)+(-358kJ)]](/tpl/images/0550/7790/da328.png)
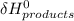 - ∑
- ∑ 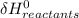
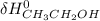 - ∑
- ∑