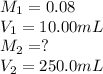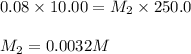Chemistry, 17.03.2020 20:12 Ezekielcassese
A solution is prepared by dissolving 0.7234 g oxalic acid (H2C2O4) in enough water to make 100.0 mL of solution. A 10.00-mL aliquot (portion) of this solution is then diluted to a final volume of 250.0 mL. What is the final molarity of the diluted oxalic acid solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3


Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
A solution is prepared by dissolving 0.7234 g oxalic acid (H2C2O4) in enough water to make 100.0 mL...
Questions


Business, 11.11.2020 04:10

Mathematics, 11.11.2020 04:10

Health, 11.11.2020 04:10



Mathematics, 11.11.2020 04:10





Social Studies, 11.11.2020 04:10

History, 11.11.2020 04:10

Chemistry, 11.11.2020 04:10





Chemistry, 11.11.2020 04:10

Mathematics, 11.11.2020 04:10

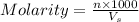
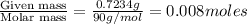
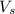 = volume of solution in ml
= volume of solution in ml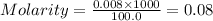
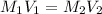
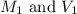 are the molarity and volume of concentrated oxalic acid solution.
are the molarity and volume of concentrated oxalic acid solution.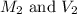 are the molarity and volume of diluted oxalic acid solution.
are the molarity and volume of diluted oxalic acid solution.