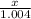Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Calculate the mass of hydrogen formed when 27 g of aluminum reacts with excess hydrochloric acid acc...
Questions

Mathematics, 24.05.2021 06:40

Mathematics, 24.05.2021 06:40

Computers and Technology, 24.05.2021 06:40

English, 24.05.2021 06:40

Health, 24.05.2021 06:40


Advanced Placement (AP), 24.05.2021 06:40

Mathematics, 24.05.2021 06:40



Chemistry, 24.05.2021 06:40

Spanish, 24.05.2021 06:40



Mathematics, 24.05.2021 06:40


Mathematics, 24.05.2021 06:40

Mathematics, 24.05.2021 06:40

Mathematics, 24.05.2021 06:40

Chemistry, 24.05.2021 06:40

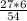 = 3 g of H₂.
= 3 g of H₂.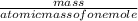
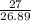
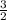 =
=