
Chemistry, 17.03.2020 21:23 kaiyah2021
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equation: 2LiOH + CO 2 Li 2 CO 3 + H 2 O. Determine the mass of carbon dioxide removed if the space vehicle carries 42.0 mol of LiOH.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

You know the right answer?
Space vehicles use solid lithium hydroxide to remove exhaled carbon dioxide according to the equatio...
Questions


Social Studies, 08.01.2020 21:31


History, 08.01.2020 21:31









Physics, 08.01.2020 21:31


Mathematics, 08.01.2020 21:31

Mathematics, 08.01.2020 21:31


Social Studies, 08.01.2020 21:31


Social Studies, 08.01.2020 21:31

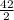 = 21moles of CO₂
= 21moles of CO₂


