
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? The reaction described in Part A required 3.36 L of calcium chloride. What is the concentration of this calcium chloride solution?
Please explain, I don't know where to begin.
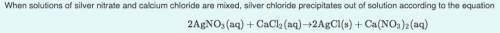

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? Th...
Questions

Physics, 13.04.2020 22:40



Business, 13.04.2020 22:40

Mathematics, 13.04.2020 22:40




Social Studies, 13.04.2020 22:41

Physics, 13.04.2020 22:41

Mathematics, 13.04.2020 22:41


Mathematics, 13.04.2020 22:41

Mathematics, 13.04.2020 22:41

Mathematics, 13.04.2020 22:41



Mathematics, 13.04.2020 22:41

Physics, 13.04.2020 22:41

Mathematics, 13.04.2020 22:41



