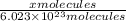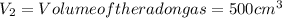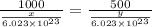Chemistry, 17.03.2020 22:04 sidneydominguez2323
1000 cm3 of hydrogen gas (hydrogen molecules, H2) contains x number of molecules at room temperature and pressure. Determine the number of atoms in 500 cm3 of radon gas (radon atoms) at the same temperature and pressure

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
1000 cm3 of hydrogen gas (hydrogen molecules, H2) contains x number of molecules at room temperature...
Questions

Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00



Mathematics, 09.01.2021 14:00


Mathematics, 09.01.2021 14:00

Biology, 09.01.2021 14:00

World Languages, 09.01.2021 14:00



Mathematics, 09.01.2021 14:00




Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00


Mathematics, 09.01.2021 14:00

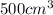 of radon gas (radon atoms) at the same temperature and pressure is
of radon gas (radon atoms) at the same temperature and pressure is 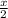
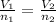
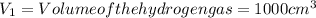
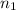 = moles of hydrogen gas =
= moles of hydrogen gas =