Chemistry, 17.03.2020 22:05 ellareynolds2337
How many liters of hydrogen gas will be produced at 280.0 K and 96.0 kPa if 40.0 grams of sodium react with excess hydrochloric acid? Write the balanced equation first.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
How many liters of hydrogen gas will be produced at 280.0 K and 96.0 kPa if 40.0 grams of sodium rea...
Questions

Mathematics, 27.09.2019 22:30





Biology, 27.09.2019 22:30




Business, 27.09.2019 22:30

Mathematics, 27.09.2019 22:30

Biology, 27.09.2019 22:30


Mathematics, 27.09.2019 22:30


Mathematics, 27.09.2019 22:30

Chemistry, 27.09.2019 22:30


Health, 27.09.2019 22:30

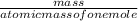
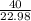
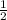 =
=