Chemistry, 18.03.2020 00:50 loganparrish2488
Select the correct answer,
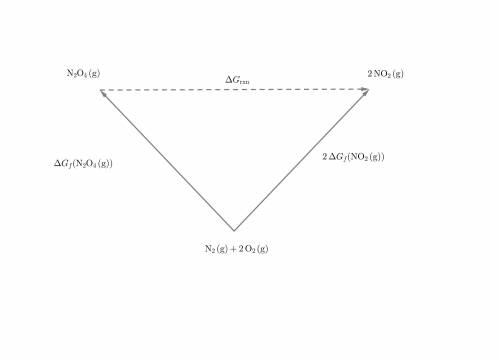
What is the AG for the following reaction at 25°C?
N204(8) 2N02(9)
Given:
N20409): AG= 98.28 kJ/mol
NO2(9): AG= 51.8 kJ/mol
OA 5.32 kJ/mol
OB. 10.64 kJ/mol
OC -5.32 kj/mol
OD. -10.64 kJ/mol


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Select the correct answer,
What is the AG for the following reaction at 25°C?
N204(8) 2N...
What is the AG for the following reaction at 25°C?
N204(8) 2N...
Questions

Biology, 04.11.2020 03:30

Mathematics, 04.11.2020 03:30


SAT, 04.11.2020 03:30


Arts, 04.11.2020 03:30




Mathematics, 04.11.2020 03:30


Mathematics, 04.11.2020 03:30

Mathematics, 04.11.2020 03:30

Advanced Placement (AP), 04.11.2020 03:30

Chemistry, 04.11.2020 03:30



English, 04.11.2020 03:30

English, 04.11.2020 03:30

Mathematics, 04.11.2020 03:30

 .
.  can be found from the Gibbs Energy of formation
can be found from the Gibbs Energy of formation  :
: .
. .
. molecules on the product side of this reaction, the
molecules on the product side of this reaction, the  to
to  . To find that value, invert
. To find that value, invert 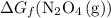 (the arrow on the left-hand side) and add that to
(the arrow on the left-hand side) and add that to  (the arrow on the right-hand side.) The resultant value would be the dashed arrow, which stands for
(the arrow on the right-hand side.) The resultant value would be the dashed arrow, which stands for  .
.