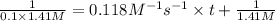At a certain temperature this reaction follows second-order kinetics with a rate constant of 0.118 M^-1* s^-1:
ClCH2CH2Cl (g) > CH2CHCI(g) + HCl (g)
a) Suppose a vessel contains ClCH2CH2Cl at a concentration of 1.41 M. Calculate how long it takes for the concentration of CICH2CH2Cl to decrease to 10.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 0.118 M...
Questions

English, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10







Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10


Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10



Mathematics, 19.05.2021 01:10

Health, 19.05.2021 01:10

Health, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

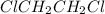 to decrease to 10.0% of its initial value.
to decrease to 10.0% of its initial value.![[A_o]=1.41 M](/tpl/images/0551/6145/c4ebe.png)
![[A]=10\%of [A_o]=0.1[A_o]](/tpl/images/0551/6145/99a59.png)
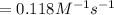
![\frac{1}{[A]}=kt+\frac{1}{[A_o]}](/tpl/images/0551/6145/a4900.png)