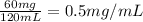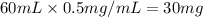Chemistry, 18.03.2020 18:54 esanchez2002fcb
G During a coarse titration, you placed 60 mg of unknown acid in 120 mL water, and dispensed 20 mL of NaOH to this acid solution (analyte) to reach the endpoint. What should be the mass and volume of the analyte so it takes 10 mL of NaOH to reach the endpoint

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
G During a coarse titration, you placed 60 mg of unknown acid in 120 mL water, and dispensed 20 mL o...
Questions


History, 13.01.2021 19:20

Mathematics, 13.01.2021 19:20


History, 13.01.2021 19:20









History, 13.01.2021 19:20

History, 13.01.2021 19:20





Mathematics, 13.01.2021 19:20

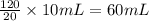 unknown acid solution.
unknown acid solution.