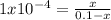Chemistry, 18.03.2020 19:39 JellalFernandes
A reaction A(g)⇌B(g)has an equilibrium constant of 1.0×10−4 For which of the initial reaction mixtures is the small approximation most likely to apply? Explain why.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
A reaction A(g)⇌B(g)has an equilibrium constant of 1.0×10−4 For which of the initial reaction mixtur...
Questions

Social Studies, 03.12.2021 23:20

Spanish, 03.12.2021 23:20


Mathematics, 03.12.2021 23:20







Mathematics, 03.12.2021 23:20


Arts, 03.12.2021 23:20

Physics, 03.12.2021 23:20

Chemistry, 03.12.2021 23:20





 in this case would be 0.00001M which is the smallest one from the given options, considering:
in this case would be 0.00001M which is the smallest one from the given options, considering: